Piccole e grandi idee di marketing che hanno permesso di gestire al meglio la pandemia Covid-19

e risparmiare soldi al contempo
Return on Investment of the COVID-19 Vaccination Campaign in New York City
This decision analytical model estimates the direct and indirect costs as well as the benefits associated with a citywide COVID-19 vaccination initiative in New York City.
Key Points
Question
Did the economic savings accrued from reductions in COVID-19 cases, hospitalizations, and deaths outweigh the investment in the COVID-19 vaccination program in New York City?
Findings
In this decision analytical model study of a citywide initiative, every $1 invested in the New York City vaccination campaign yielded an estimated $10.19 in cost savings from lower infection and mortality rates, fewer productivity losses, and averted health care use.
Meaning
Findings of this study suggest that COVID-19 vaccination in New York City was associated with reduction in severe outcomes and avoidance of substantial economic losses.
Abstract
Importance
New York City, an early epicenter of the pandemic, invested heavily in its COVID-19 vaccination campaign to mitigate the burden of disease outbreaks. Understanding the return on investment (ROI) of this campaign would provide insights into vaccination programs to curb future COVID-19 outbreaks.
Objective
To estimate the ROI of the New York City COVID-19 vaccination campaign by estimating the tangible direct and indirect costs from a societal perspective.
Design, Setting, and Participants
This decision analytical model of disease transmission was calibrated to confirmed and probable cases of COVID-19 in New York City between December 14, 2020, and January 31, 2022. This simulation model was validated with observed patterns of reported hospitalizations and deaths during the same period.
Exposures
An agent-based counterfactual scenario without vaccination was simulated using the calibrated model.
Main Outcomes and Measures
Costs of health care and deaths were estimated in the actual pandemic trajectory with vaccination and in the counterfactual scenario without vaccination. The savings achieved by vaccination, which were associated with fewer outpatient visits, emergency department visits, emergency medical services, hospitalizations, and intensive care unit admissions, were also estimated. The value of a statistical life (VSL) lost due to COVID-19 death and the productivity loss from illness were accounted for in calculating the ROI.
Results
During the study period, the vaccination campaign averted an estimated $27.96 (95% credible interval [CrI], $26.19-$29.84) billion in health care expenditures and 315 724 (95% CrI, 292 143-340 420) potential years of life lost, averting VSL loss of $26.27 (95% CrI, $24.39-$28.21) billion. The estimated net savings attributable to vaccination were $51.77 (95% CrI, $48.50-$55.85) billion. Every $1 invested in vaccination yielded estimated savings of $10.19 (95% CrI, $9.39-$10.87) in direct and indirect costs of health outcomes that would have been incurred without vaccination.
Conclusions and Relevance
Results of this modeling study showed an association of the New York City COVID-19 vaccination campaign with reduction in severe outcomes and avoidance of substantial economic losses. This significant ROI supports continued investment in improving vaccine uptake during the ongoing pandemic.
Introduction
The emergence of SARS-CoV-2 spurred unprecedented global efforts toward the development of vaccines to contain the COVID-19 pandemic. In the first year of the pandemic, 2 highly effective messenger RNA (mRNA) vaccines (mRNA-1273 [Moderna Spikevax] and BNT162b2 [Pfizer/BioNTech Comirnaty]) and 1 adenovirus-based vaccine (Ad26.COV2.S [Janssen]) were developed and received emergency use authorization in the US. New York City, an early epicenter of the global spread of COVID-19 and the largest metropolitan area in the US, launched its mass vaccination campaign on December 14, 2020. By January 31, 2022, 84.9% of New York City residents had received at least 1 dose of the authorized vaccines.1
Vaccination has been associated with reduced burden of COVID-19,2,3,4,5,6 in particular prevention of severe illness and death.7,8,9 In addition to its role in direct protection of vaccinated individuals against SARS-CoV-2 infection and severe outcomes, vaccination is associated with the societal benefits of preventing productivity losses, reducing the need for social-distancing measures, and providing indirect protection to unvaccinated individuals through herd immunity. A previous study estimated that the NYC Vaccine for All Campaign reduced disease burden substantially.2 This public health success required considerable financial investment to acquire and deliver vaccines, promote vaccination to the public, and address vaccine hesitancy in many communities. Understanding the return on this investment would provide important insights into vaccination programs to quell future COVID-19 outbreaks.
In this decision analytical model study, we aimed to estimate the return on investment (ROI) of the New York City COVID-19 vaccination campaign by estimating the tangible direct and indirect costs from a societal perspective. To conduct this analysis, we compared costs and outcomes of the actual pandemic trajectory with vaccination in New York City with those of a counterfactual scenario without vaccination whose outcomes were derived from an agent-based simulation model of COVID-19 transmission dynamics; this model was calibrated to data for the burden of disease in New York City.
Methods
Because data were not collected specifically for this decision analytical model study and no identifiable personal data were used, specific ethical approval and informed consent were not required in accordance with York University research ethics guidelines for program evaluation activities relying on secondary use of anonymous data. We followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.10
Study Design and Simulation Modeling Framework
To estimate the ROI for the New York City COVID-19 vaccination campaign from December 14, 2020, to January 31, 2022, we integrated into the study design both direct and indirect costs associated with vaccination and health outcomes. All costs were calculated and reported in 2021 US dollars.
Direct costs of vaccination, as part of the initial value of investment (IVI), were provided by the New York City Department of Health and Mental Hygiene (DOHMH). For direct health outcomes, we identified the costs of outpatient visits, emergency department (ED) visits, emergency medical services (EMS) calls, hospitalizations, and intensive care unit (ICU) admissions as well as the value of a statistical life (VSL) lost due to death from COVID-19. Indirect health outcomes included workdays and production losses due to sickness and adverse reactions to vaccination. We also accounted for the indirect costs associated with COVID-19 deaths by calculating potential years of life lost (PYLL) using the VSL, a measure of individuals’ willingness to pay for mortality risk reductions.
To calculate the total costs for the final value of investment (FVI) associated with health outcomes in the actual pandemic trajectory with vaccination and counterfactual scenario without vaccination, we calibrated an agent-based simulation model to probable and confirmed cases of COVID-19 in New York City while accounting for disease characteristics and timelines of different SARS-CoV-2 variants identified in New York City, including Iota (B.1.526), Alpha (B.1.1.7), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529), in addition to Wuhan-Hu-I, the original pandemic strain (eMethods, eTables 1-5, and eFigures 1 and 2 in the Supplement).
Investment in the Vaccination Campaign
We estimated direct government investment in the vaccination campaign using data provided by the DOHMH (Table 1).11,12,13,14,15 These data included the state and federal funding that came through the DOHMH. Direct costs included the purchase value of vaccine doses over the study period and costs of the vaccination clinic setup; advertisement and outreach; and vaccine transportation, storage, and administration. In calculating unit vaccine costs, we accounted for a 0.25% wastage rate provided by the DOHMH.
Table 1. Direct and Indirect Costs of the New York City Vaccination Campaign, With Estimates of Adverse Reactions in Vaccine Recipients.
| Investment toward vaccination | Estimate | Source |
|---|---|---|
| Direct cost, $a | ||
| Advertisement and outreach | 243 390 758.20 | New York City DOHMH |
| Vaccination clinic setup | 4 344 813.00 | |
| Vaccine storage and transportation | 7 205 179.89 | |
| Vaccine administration | 1 872 058 070.42 | |
| CIR DOITT–developed applications | 9 500 000.00 | |
| Mobile and homebound vaccinations | 21 400 000.00 | |
| Vaccine doses | 282 663 374.80 | |
| Vaccine wastage | ||
| Overall % | 0.25 | New York City DOHMH |
| Indirect cost, % | ||
| % Of vaccinated employed adultsb | 71.8 | US Census Bureau11, 2022 |
| Workdays lost due to visiting vaccination clinic | 0.5 | Bologna et al12, 2021 |
| % Of Spikevax (Moderna) recipients with adverse reactions | ||
| After the first dose | 51.7 | Chapin-Bardales et al13, 2021 |
| After the second dose | 74.8 | |
| % Of Comirnaty (Pfizer/BioNTech) recipients with adverse reactions | ||
| After the first dose | 48.0 | Chapin-Bardales et al13, 2021 |
| After the second dose | 64.2 | |
| % Of Ad26.COV2.S vaccine (Janssen) recipients with adverse reactions | 76.0 | Shay et al14, 2021 |
| Workdays lost due to adverse reactions | Levi et al15, 2021 | |
| After the first dose | 1.7 | |
| After the second dose | 1.4 |
Abbreviations: CIR, Citywide Immunization Registry; DOHMH, Department of Health and Mental Hygiene; DOITT, Department of Information Technology and Telecommunications.
a
Costs are in 2021 US dollars.
b
In the New York City metropolitan area.
Indirect costs of vaccination included workdays lost for visiting vaccination clinics and loss of productivity due to adverse reactions to vaccines.15 We used published estimates for the prevalence of adverse outcomes after vaccination.16,17 Costs related to workdays lost were estimated using the percentage of vaccinated adults who were employed and per capita personal income of $74 472 for 2020.18
Costs of COVID-19 Health Outcomes
We used estimates of COVID-19 symptomatic infections and hospitalizations from the projections of the agent-based simulation model to calculate direct costs (ie, treatment and transportation costs) and indirect costs (workdays lost) associated with illness and hospitalizations (Table 2).19,20,21,22,23,24,25,26,27,28,29Costs of health outcomes were stratified into outpatient visits for symptomatic infection, hospitalizations, and/or intensive care for severe illness, EMS calls, and ED visits. Costs of testing for SARS-CoV-2 infection for individuals who were hospitalized and those who received ED care were included in estimates of the total costs per patient. Transportation costs were estimated by multiplying the mean cost per round trip to a health care facility by the number of care-seeking cases and the number of visits.
Table 2. Direct and Indirect Costs and Units Associated With Health Outcomes of COVID-19 Illness.
| Outcomes of COVID-19 illness | Estimate | Source |
|---|---|---|
| Direct cost, $ | ||
| Outpatient treatment per visit | 1020.10 | FAIR Health19a |
| Round trip to health care facility for outpatient treatment | 44.49 | Kuhmerker et al20, 2010 |
| Hospitalization (mean per patient) | ||
| Without ICU | 39 499.18 | FAIR Health19a |
| With ICU | 113 249.31 | FAIR Health19a |
| ED care (mean per visit) | 3305.01 | Castlight Health21 |
| EMS transportation | 900.00 | Campanile22, 2020 |
| Direct cost | ||
| Mean No. of outpatient visits per symptomatic nonhospitalized case | 0.5 | Author assumption |
| No. of ED visits for nonhospitalized severe cases | 1.0 | Author assumption |
| No. of EMS calls vs No. of hospitalized patients | 2.5 | Xie et al23, 2021 |
| Indirect cost, d | ||
| Workdays lost for symptomatic nonhospitalized cases | 10.0 | NYC Health24 |
| Symptomatic days before hospitalization | 3.5 | Iuliano et al25, 2022; Christensen et al26, 2022; Nguyen et al27, 2021 |
| Median duration of hospital stay | ||
| Without ICU for patients with SARS-CoV-2 non-Omicron variant infection | 6.0 | |
| With ICU for patients with SARS-CoV-2 non-Omicron variant infection | 15.0 | |
| Without ICU for patients with SARS-CoV-2 Omicron variant infection | 3.0 | |
| With ICU for patients with SARS-CoV-2 Omicron variant infection | 7.0 |
Abbreviations: ED, emergency department; EMS, emergency medical services; ICU, intensive care unit.
a
Cost estimates were adjusted due to the 8.3% higher health care costs in New York City than in New York state.28,29
Nonhospitalized symptomatic cases among working adults were assumed to have a 10-day isolation period after the onset of symptoms,24 which was included in the calculation of workdays lost. For hospitalized COVID-19 cases, we calculated workdays lost by taking into account the duration of hospital stay with or without ICU admission, which was derived from the simulation model projections using published estimates.25,26,27
Costs Associated With Potential Years of Life Lost
We used the VSL to calculate the costs of PYLL attributed to COVID-19 deaths. The PYLL for each COVID-19 death was calculated using the life expectancy table. The mean VSL for the US population is estimated to vary between $5.3 million and $17.3 million,30,31,32,33 with the value of a statistical life-year (VSLY) having a large range depending on the mean life expectancy for an adult.31,32,34The VSLY represents society’s willingness to pay for 1 year of life. We considered the conservative value of $100 000 to be within the range of conventional benchmarks per quality-adjusted life-year in the US, with an annual discount rate (r) of 3% that is consistent with guidelines developed for the US.35,36 Using the age stratification of COVID-19 deaths obtained from the DOHMH,37 we calculated the total discounted VSL loss38 as follows: VSL loss = VSLY/r − [1/(1 + r)PYLL] (VSLY/r).
Return on Investment Analysis
We estimated the ROI as follows: ROI = [(FVI − IVI)/IVI] × 100%. The IVI included direct and indirect costs of the vaccination campaign in New York City (Table 1). The FVI was calculated using the difference between total (direct and indirect) costs of health outcomes in the actual pandemic trajectory and those of the counterfactual scenario without vaccination (Table 2). In the counterfactual scenario, we excluded from the simulation model the effectiveness of vaccines against infection, symptomatic infection, and severe disease, and we removed the government and societal investment toward the vaccination campaign.
Statistical Analysis
The agent-based simulation model was calibrated with 500 independent Monte Carlo realizations and simulated the counterfactual scenario without vaccination (eMethods in the Supplement). For each independent Monte Carlo realization, we calculated the PYLL and VSL lost from the simulated health outcomes and derived the 95% credible intervals (CrIs) for the ROI estimates using a bias-corrected and accelerated bootstrap method with 500 replications. The computational model was implemented in Julia language. The statistical analysis of ROI was conducted in R, version 4.1.3 (R Foundation for Statistical Computing). For hypothesis testing, 2-sided P = .05 was considered to be significant.
Results
The estimated direct costs of total investment in the vaccination campaign were $2.44 billion between December 14, 2020, and January 31, 2022 (Table 1). Indirect costs of vaccination (Tables 1 and 2), including loss of workdays due to visiting vaccination clinics and adverse reactions to vaccines, were estimated to be $2.39 billion.
The direct costs of COVID-19 health outcomes (ie, hospitalizations, outpatient treatments, ED care, and EMS calls) were estimated at $7.45 (95% CrI, $7.15-$7.79) billion with vaccination and $33.04 (95% CrI, $31.43-$34.79) billion without vaccination during the study period (Figure 1A). Indirect costs associated with loss of workdays due to disease outcomes (ie, days of isolation for symptomatic cases and/or hospitalization) were $1.87 (95% CrI, $1.79-$1.99) billion with vaccination and $4.22 (95% CrI, $4.03-$4.41) billion without vaccination. Overall, vaccination was estimated to have averted $27.96 (95% CrI, $26.19-$29.84) billion of additional direct and indirect health care costs that would have incurred without vaccination.
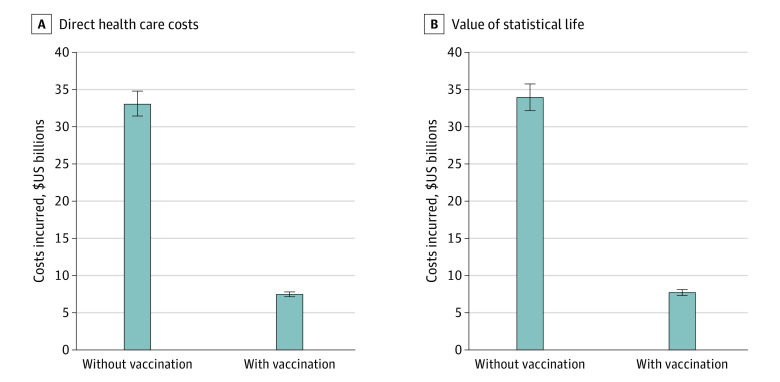
We estimated that the pandemic resulted in 92 280 (95% CrI, 86 806-97 083) PYLL in New York City during the study period (eFigure 3 in the Supplement), with a total VSL loss of $7.70 (95% CrI, $7.30-$8.11) billion (Figure 1B). The mean PYLL was 6.06 (95% CrI, 4.39-9.59) per person. Without vaccination, the total PYLL would have been significantly higher (408 707 [95% CrI, 388 407-430 039]; P < .001 [based on the Kruskal-Wallis test by ranks]) (eFigure 3 in the Supplement), leading to a substantially larger VSL loss of $33.93 (95% CrI, $32.16-$35.75) billion (Figure 1B). A total of 315 724 (95% CrI, 292 143-340 420) PYLL was averted by vaccination. Thus, the vaccination campaign yielded net VSL savings of $26.27 (95% CrI, $24.39-$28.21) billion, which was equivalent to savings of $0.55 (95% CrI, $0.51-$0.59) million per averted death. The largest PYLL averted was among individuals 50 years or older (eFigure 3 in the Supplement).
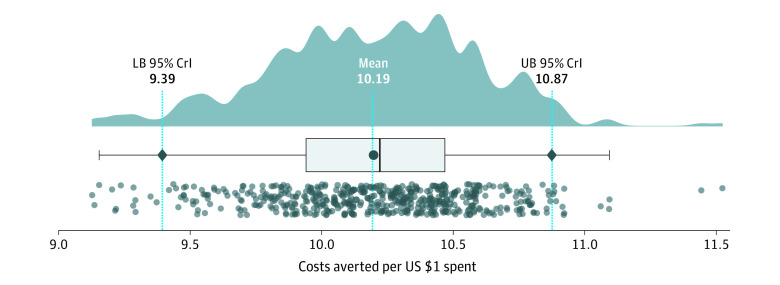
From a societal perspective, the ROI was estimated at 1019% (95% CrI, 939%-1087%), yielding equivalent savings of $10.19 (95% CrI, $9.39-$10.87) in costs of health outcomes for every $1 spent toward both direct and indirect costs of vaccination in New York City (Figure 2). The net savings achieved by the vaccination campaign amounted to $51.77 (95% CrI, $48.50-$55.85) billion.
We also conducted the ROI analysis by excluding the VSL. From a societal perspective, the ROI without VSL was estimated at 427% (95% CrI, 391%-469%), which was equivalent to savings of $4.27 (95% CrI, $3.91-$4.69) in direct and indirect health care costs for every $1 spent toward vaccination in New York City.
Discussion
To our knowledge, this study was the first ROI analysis of the COVID-19 vaccination campaign in New York City. We estimated the direct and indirect costs as well as the benefits of this vaccination campaign in terms of averted health care costs and VSL from December 14, 2020, to January 31, 2022. The results suggest that the investment in vaccination has had a substantial ROI from a societal perspective.
The findings should be interpreted in the context of a population study. Promoting vaccine uptake was the core strategy of the DOHMH to address SARS CoV-2 transmission, preserve hospital capacity, and mitigate the disease burden while resuming economic, educational, and social activities. The scale of investment in COVID-19 vaccination was associated with a comparatively more rapid pace of vaccine delivery for primary series in New York City, with 64% of the mRNA vaccines administered being from Pfizer/BioNTech, according to DOHMH data. Given the large savings in direct and indirect health care costs associated with vaccination and the comparable estimates of effectiveness for the Comirnaty and Spikevax vaccines, we do not expect the price difference between the 2 vaccines or their proportion in the New York City vaccination campaign to significantly alter the ROI estimates. However, the pace of vaccine distribution and vaccination coverage, which varies across different population settings, remains an important factor in the ROI analysis.
Limitations
This study has several limitations. First, the ROI analysis did not capture all costs of the vaccination campaign, including those incurred by pharmacies and other city agencies beyond the DOHMH. Second, in estimating the health care costs under the counterfactual scenario without vaccination, we did not account for the costs that would have been incurred for implementing nonpharmaceutical interventions, such as potential lockdowns, business closure or reduced capacity, and school closures. Some of the additional costs of such interventions may be offset by alleviating health outcomes, which were associated with reduced disease burden. We also did not take into account the association between vaccination and the economic burden from displacement of treatment and delivery of regular health services. We could not ascertain the proportion of symptomatic outpatients who were tested for SARS-CoV-2 infection, and thus we did not include the associated costs in the ROI analysis. Without vaccination, this proportion could have been substantially higher, leading to additional direct costs.
Third, we assumed that there was no limit to health care capacity (eg, hospital and ICU beds), and thus there were no constraints for health care spending, in the counterfactual scenario without vaccination. Without vaccination, the health care system could have been overwhelmed with individuals with COVID-19, potentially triggering triage policies for delivery of care with increased burden of disease and VSL losses. Fourth, we did not account for certain current and future benefits of vaccination, including averting long COVID, resuming operation of businesses, holding in-person learning at schools, and providing essential services.
Most of these limitations, except our inability to capture all costs of the vaccination campaign, likely underestimated the ROI. Therefore, the results may be conservative regarding the benefits of the vaccination program in the city.
Conclusions
In this decision analytical model study, the New York City COVID-19 vaccination campaign was found to be associated with not only reduction in severe outcomes but also avoidance of substantial economic losses from high costs of health care and value of lives lost. This considerable ROI provides compelling evidence for the continued investment in improving vaccine uptake during the ongoing COVID-19 pandemic.
Supplement.
eMethods.
eTable 1. Mixing Patterns and the Daily Number of Contacts Derived From Empirical Observations
eTable 2. Estimated Vaccine Efficacies (%) and Their 95% Confidence Intervals From Published Studies for Pfizer-BioNTech Vaccines
eTable 3. Estimated Vaccine Effectiveness (%) and Their 95% Confidence Intervals From Published Studies for Moderna Vaccines
eFigure 1. Temporal Relative Effectiveness of Vaccines Against Infection and Severe Disease Derived From Gaussian Fit to Data of Vaccine Effectiveness After the Second Dose Prior to Omicron
eTable 4. Percentage of Seropositivity Estimated in Different Age Groups by October 1, 2020
eFigure 2. Model Fit to Probable and Confirmed Cases of COVID-19 in NYC and Projections of Cases per 100,000 Population in NYC With and Without Vaccination (Counterfactual Scenario) From October 1, 2020 to January 31, 2022
eTable 5. Reported (Pandemic Burden in NYC During Vaccination Campaign) and Simulated (Without Vaccination) Deaths and Hospitalizations From December 14, 2020 to January 31, 2022
eFigure 3. Estimated Potential Years of Life Lost (PYLL)
eReferences
References
- 1.NYC Health. COVID-19: data. Vaccines. Accessed March 24, 2022. https://www1.nyc.gov/site/doh/covid/covid-19-data-vaccines.page
- 2.Shoukat A, Vilches TN, Moghadas SM, et al. Lives saved and hospitalizations averted by COVID-19 vaccination in New York City: a modeling study. Lancet Reg Health Am. 2022;5:100085. doi: 10.1016/j.lana.2021.100085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moghadas SM, Vilches TN, Zhang K, et al. The impact of vaccination on Coronavirus Disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis. 2021;73(12):2257-2264. doi: 10.1093/cid/ciab079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Cantor J, Simon KI, Bento AI, Wing C, Whaley CM. Vaccinations against COVID-19 may have averted up to 140,000 deaths in the United States. Health Aff (Millwood). 2021;40(9):1465-1472. doi: 10.1377/hlthaff.2021.00619[DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilches TN, Moghadas SM, Sah P, et al. Estimating COVID-19 infections, hospitalizations, and deaths following the US vaccination campaigns during the pandemic. JAMA Netw Open. 2022;5(1):e2142725. doi: 10.1001/jamanetworkopen.2021.42725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sah P, Vilches TN, Moghadas SM, et al. Accelerated vaccine rollout is imperative to mitigate highly transmissible COVID-19 variants. EClinicalMedicine. 2021;35:100865. doi: 10.1016/j.eclinm.2021.100865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293-1302. doi: 10.1016/S1473-3099(22)00320-6[DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steele MK, Couture A, Reed C, et al. Estimated number of COVID-19 infections, hospitalizations, and deaths prevented among vaccinated persons in the US, December 2020 to September 2021. JAMA Netw Open. 2022;5(7):e2220385. doi: 10.1001/jamanetworkopen.2022.20385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenforde MW, Self WH, Gaglani M, et al. ; IVY Network . Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death: United States, March 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(12):459-465. doi: 10.15585/mmwr.mm7112e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husereau D, Drummond M, Augustovski F, et al. Correction to: Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated Reporting Guidance for Health Economic Evaluations. Appl Health Econ Health Policy. 2022;20(5):781-782. doi: 10.1007/s40258-022-00743-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Census Bureau . Week 39 Household Pulse Survey: September 29 – October 11. January 5, 2022. Accessed March 20, 2022. https://www.census.gov/data/tables/2021/demo/hhp/hhp39.html
- 12.Bologna JM, Greenberg RI, Skubas SR, Shapiro HS. New York employees are entitled to paid time off for COVID-19 vaccinations. March 18, 2021. Accessed March 20, 2022. https://www.shrm.org/ResourcesAndTools/legal-and-compliance/state-and-local-updates/Pages/New-York-Employees-Are-Entitled-to-Paid-Time-Off-for-COVID-19-Vaccinations.aspx
- 13.Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325(21):2201-2202. doi: 10.1001/jama.2021.5374 [DOI] [PubMed] [Google Scholar]
- 14.Shay DK, Gee J, Su JR, et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine: United States, March-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680-684. doi: 10.15585/mmwr.mm7018e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levi ML, McMillan D, Dhandha V, Allan J, D’ercole F. COVID-19 mRNA vaccination, reactogenicity, work-related absences and the impact on operating room staffing: a cross-sectional study. Perioper Care Oper Room Manag. 2021;25:100220. doi: 10.1016/j.pcorm.2021.100220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statista. Personal income in New York: income per capita 2000-2021. Accessed March 24, 2022. https://www.statista.com/statistics/205461/per-capita-personal-income-in-new-york/
- 19.FAIR Health. States by the numbers. Accessed March 21, 2022. https://www.fairhealth.org/states-by-the-numbers
- 20.Kuhmerker K, Pohl MB, Park C, Choudhry K. Medicaid transportation in New York—background and options: Medicaid Institute at United Hospital Fund. 2010. Accessed June 5, 2022. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/4404.pdf
- 21.Castlight Health . The costs of COVID-19: how much does it really cost to seek care? Castlight Health. Accessed June 5, 2022. https://www.castlighthealth.com/wp-content/uploads/2020/03/Costs-of-COVID-19.pdf
- 22.Campanile C. NYC ambulance rides are about to cost a whole lot more—here’s why. New York Post. December 1, 2020. Accessed March 23, 2022. https://nypost.com/2020/12/01/nyc-ambulance-rides-are-about-to-cost-a-whole-lot-more/
- 23.Xie Y, Kulpanowski D, Ong J, Nikolova E, Tran NM. Predicting COVID-19 emergency medical service incidents from daily hospitalisation trends. Int J Clin Pract. 2021;75(12):e14920. doi: 10.1111/ijcp.14920 [DOI] [PubMed] [Google Scholar]
- 24.NYC Health. COVID-19: when you are sick. Accessed March 11, 2022. https://www1.nyc.gov/site/doh/covid/covid-19-symptoms-chronic-health-risks.page
- 25.Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods—United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. doi: 10.15585/mmwr.mm7104e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen PA, Olsen RJ, Long SW, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with Coronavirus disease 2019 caused by the Omicron variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642-652. doi: 10.1016/j.ajpath.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open. 2021;4(3):e210417. doi: 10.1001/jamanetworkopen.2021.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Health Care Cost Institute . Trends in total and out-of pocket spending in Metro areas: 2012-2015. Health Care Cost Institute. 2017. Accessed June 5, 2022. https://healthcostinstitute.org/images/easyblog_articles/103/OOP-Spending-Metro-Areas-Data-Brie_20200109-190735_1.pdf
- 29.Health Care Cost Institute . 2015 Health care cost and utilization report. Health Care Cost Institute. 2016. Accessed June 6, 2022. https://healthcostinstitute.org/images/pdfs/2015-HCCUR-11.22.16.pdf
- 30.Robinson LA, Sullivan R, Shogren JF. Do the benefits of COVID-19 policies exceed the costs? exploring uncertainties in the age-VSL relationship. Risk Anal. 2021;41(5):761-770. doi: 10.1111/risa.13561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ASPE. Appendix D: updating value per statistical life (VSL) estimates for inflation and changes in real income. Accessed September 30, 2022. https://aspe.hhs.gov/reports/updating-vsl-estimates
- 32.Conover C. How economists calculate the costs and benefits of COVID-19 lockdowns. Forbes Magazine. March 27, 2020. Accessed September 30, 2022. https://www.forbes.com/sites/theapothecary/2020/03/27/how-economists-calculate-the-costs-and-benefits-of-covid-19-lockdowns/
- 33.US Department of Transportation. Departmental guidance on valuation of a statistical life in economic analysis. Accessed September 30, 2022. https://www.transportation.gov/office-policy/transportation-policy/revised-departmental-guidance-on-valuation-of-a-statistical-life-in-economic-analysis
- 34.Kenkel D. Using estimates of the value of a statistical life in evaluating consumer policy regulations. J Consum Policy. 2003;26(1):1-21. doi: 10.1023/A:1022646013504 [DOI] [Google Scholar]
- 35.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 36.Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan. 2020;35(1):107-114. doi: 10.1093/heapol/czz127 [DOI] [PubMed] [Google Scholar]
- 37.NYC Health. COVID-19: data. Trends and totals. Accessed November 30, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page
- 38.Aldy JE, Viscusi WK. Age differences in the value of statistical life: revealed preference evidence. RFF Discussion Paper No. 07-05. Updated November 27, 2012. Accessed June 5, 2022. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=979003
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement.
eMethods.
eTable 1. Mixing Patterns and the Daily Number of Contacts Derived From Empirical Observations
eTable 2. Estimated Vaccine Efficacies (%) and Their 95% Confidence Intervals From Published Studies for Pfizer-BioNTech Vaccines
eTable 3. Estimated Vaccine Effectiveness (%) and Their 95% Confidence Intervals From Published Studies for Moderna Vaccines
eFigure 1. Temporal Relative Effectiveness of Vaccines Against Infection and Severe Disease Derived From Gaussian Fit to Data of Vaccine Effectiveness After the Second Dose Prior to Omicron
eTable 4. Percentage of Seropositivity Estimated in Different Age Groups by October 1, 2020
eFigure 2. Model Fit to Probable and Confirmed Cases of COVID-19 in NYC and Projections of Cases per 100,000 Population in NYC With and Without Vaccination (Counterfactual Scenario) From October 1, 2020 to January 31, 2022
eTable 5. Reported (Pandemic Burden in NYC During Vaccination Campaign) and Simulated (Without Vaccination) Deaths and Hospitalizations From December 14, 2020 to January 31, 2022
eFigure 3. Estimated Potential Years of Life Lost (PYLL)
eReferences
Articles from JAMA Network Open are provided here courtesy of American Medical Association